Research

Myelination
Myelin is formed when cells called oligodendrocytes extend processes that grow and wrap multiple times around an axon. Myelination is highly dependent on the coordinated synthesis of myelin membranes, which contain myelin-specific proteins and a unique composition of lipids compared to normal cellular membranes. Damage to myelin, or demyelination, occurs in neurological diseases like multiple sclerosis. Healthy myelin is essential for efficient neuronal signal transduction and demyelination can lead to neuron damage and degeneration.
Models of Myelination
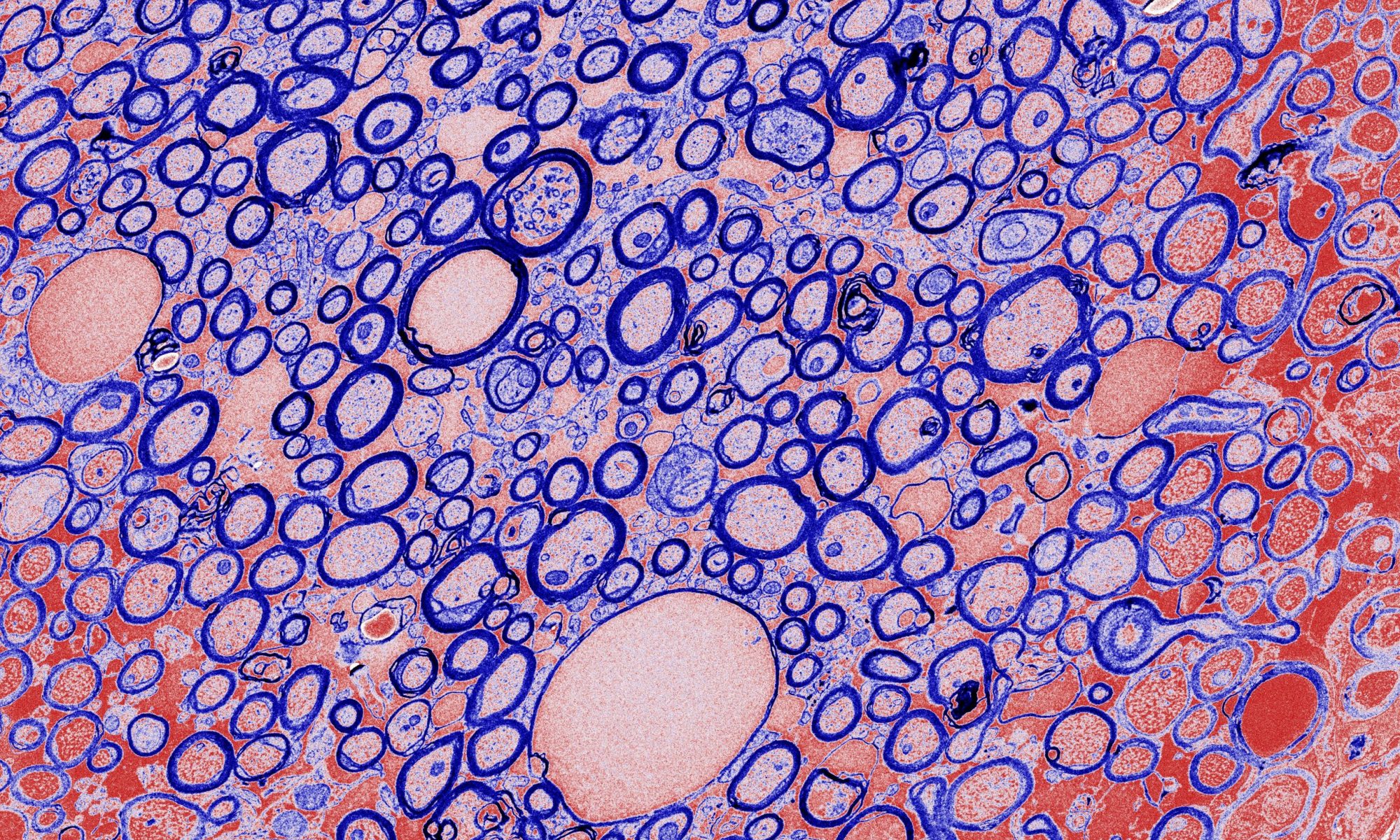
To study myelination, we employ cellular and animal models of myelination. We isolate primary oligodendrocyte precursor cells (or OPCs) from rodents as an in vitro model of myelination. OPCs can be cultured in a petri dish and differentiated into mature oligodendrocytes that form myelin-like membrane extensions. We also use toxin-based and genetic-based mouse models of demyelination to investigate how CNS tissues respond to myelin damage and to identify pathways involved in myelin repair.
Pathophysiology of Lipids in Neurological Disease
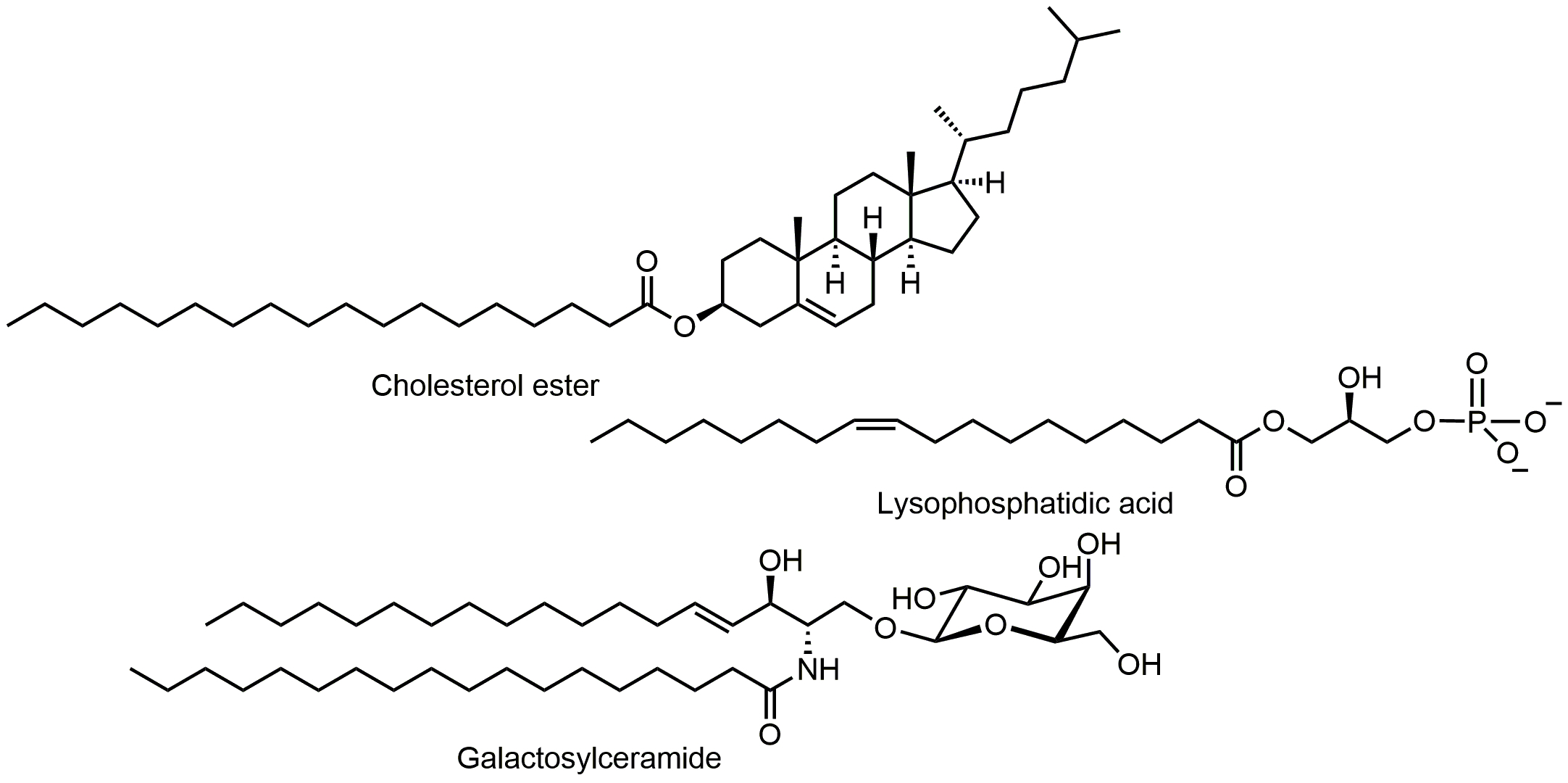
Lipids in the brain are highly abundant relative to other tissues and play unique roles specific to the central nervous system (CNS). Most CNS lipids reside in myelin membranes that insulate axons. The critical role of lipids in CNS diseases is underscored by the large family of genetic leukodystrophies (or white matter diseases) that are caused by dysregulation of lipid metabolism. One such example is X-linked adrenoleukodystrophy (X-ALD), in which elevated very long chain fatty acids (C22-C26) cause brain and spinal cord demyelination and peripheral effects on adrenal glands and testes. Lipid metabolism also plays critical roles in neurological disease of more complex etiology. For example, disruptions in cholesterol metabolism have been implicated in multiple sclerosis and Alzheimer’s disease.
Our goal is to map lipid regulatory pathways in normal CNS physiology and in CNS pathophysiology. Our initial focus examines how lipid profiles change during demyelination and remyelination, which occurs episodically during the relapsing-remitting form of multiple sclerosis. A better understanding of remyelination could lead to the identification of new targets for therapeutic intervention for people living with MS.
Roles of Hormones in Neurological Disease
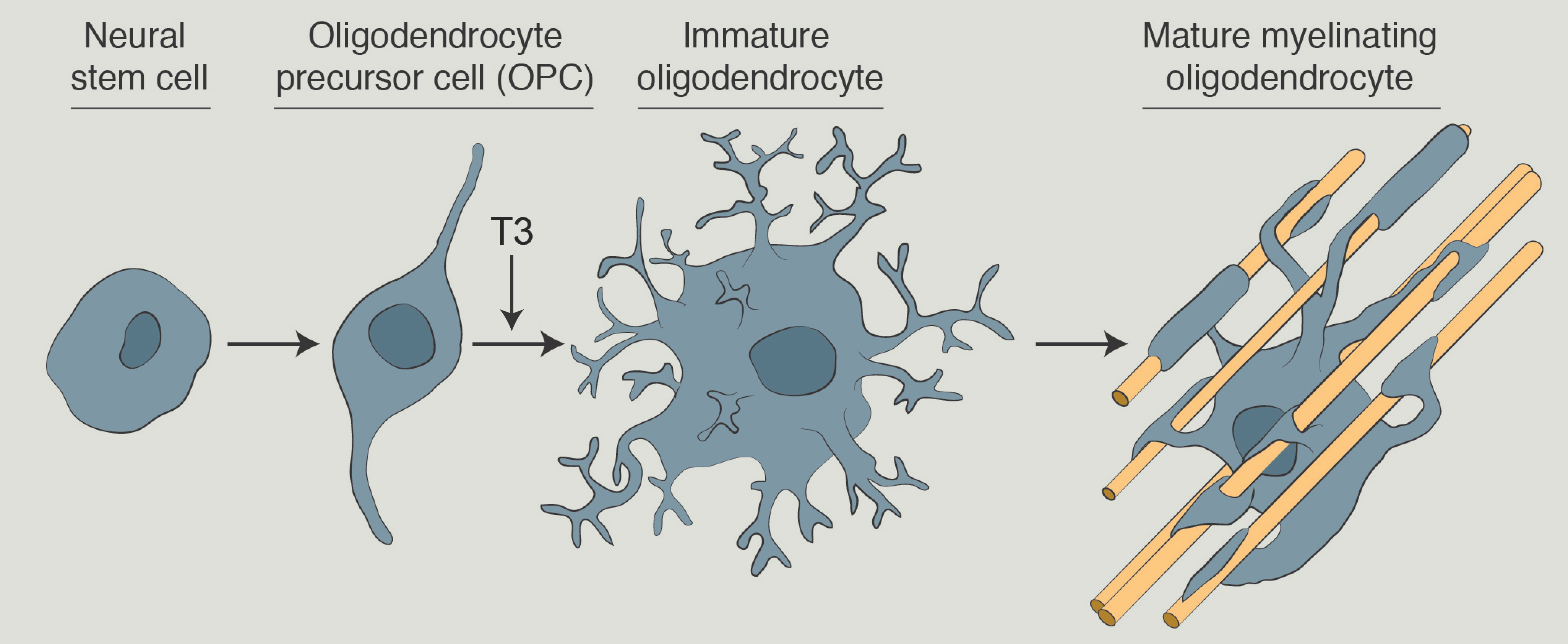
Endocrine modulators, including thyroid hormone and estrogen, are master regulators of lipid metabolism and their actions have been well characterized in peripheral tissues. Thyroid hormone and estrogen have also been shown to promote myelin repair in various models, but it is not known how these hormones are modulating the extensive lipid metabolism required for myelination. We will identify critical pathways at the intersection of endocrine regulation and neurological diseases. We will investigate these interactions in the context of diseases affected by myelin damage, and we anticipate that these findings will have implications for other neurological diseases.
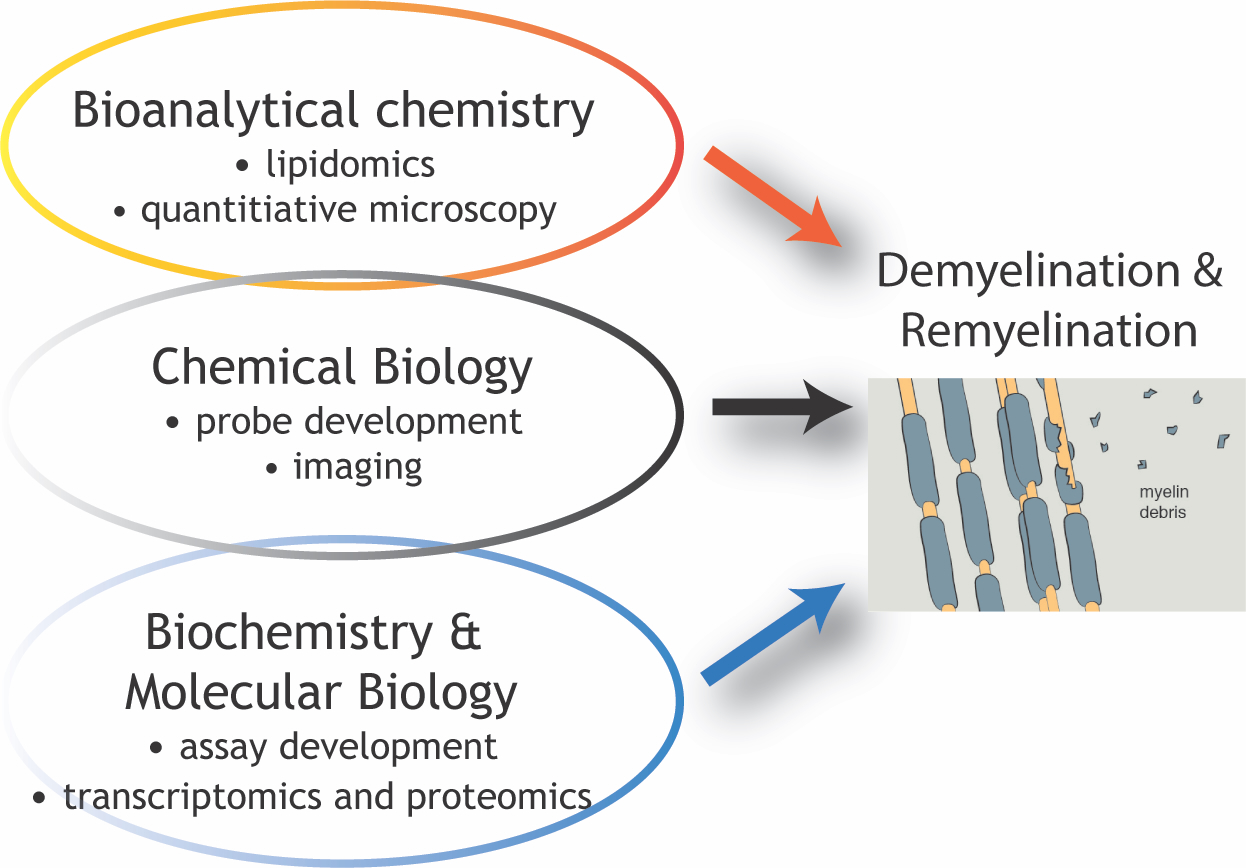
Experimental Methods
Our laboratory uses an interdisciplinary toolbox to study lipids and hormones in the context of demyelination and remyelination. We apply diverse approaches from the fields of chemical biology, bioanalytical chemistry, biochemistry, and molecular biology, including lipid mass spectrometry, transcriptomics, and flow cytometry. We also use a variety of imaging modalities, including wide-field bright-field and fluorescence imaging, spinning disk confocal microscopy, and electron microscopy.